Rehabilitation Engineering Center on Wheeled Mobility
Georgia Institute of Technology
ABSTRACT
Inventors of assistive technology often lack resources to support product development and this may hinder their ability to develop useful and commercially viable devices. The purpose of this on-going project is to support inventor and small company development of promising mobility-related technologies. Specifically, the process includes: 1) an informal brainstorming session addressing technical, market and user issues; 2) formal screening of device potential and RERC capacity to assist in development; 3) invitation to engage inventors/companies with promising technologies; and 4) product testing and/or development of promising technologies. This paper describes the project’s approach and reviews activities over the past year.
KEYWORDS:
wheelchair, seating, technology transfer, knowledge translation
Introduction and Specific Aims
In 2003, the NIDRR asked the U.S. Department of Commerce, Office of Strategic Industries and Economic Security Interagency to assess the AT industry in the US (1). The report was very comprehensive and confirmed two things that many in the AT field already know: 1) many AT devices target small markets, and 2) a lot of companies are very small with limited abilities to undertake development and testing.
The purpose of this on-going project is to support inventor and small company development of promising mobility-related technologies. The project is designed to formalize engagement of inventors and small companies to provide a basic level of support and guidance as well as more advanced development and testing to those with promising mobility products. Specifically, the process includes: 1) an informal brainstorming session addressing technical, market and user issues; 2) formal screening of device potential and RERC capacity to assist in development; 3) invitation to engage inventors/companies with promising technologies; and 4) product testing and/or development of promising technologies. This paper will describe the project approach and review activities over the past year.
Background and Rationale
The Department of Commerce reported data from 359 AT companies among which the largest subgroup (21%) listed mobility as their primary product focus. Data indicated that 60% of respondents had 10 or fewer employees. Perhaps related to this data on size, only 25% identified ‘product testing’ as an activity they undertake and this was most certainly biased to the larger companies. The report explicitly addressed the significant problems associated with limited markets for AT:
To varying degrees, the industry is thwarted by the size of product markets, which can be very small, making it difficult for companies to generate revenues sufficient to attract investors and discouraging them from making their own investments in R&D and manufacturing capacity. This challenge appears even more significant due to the small size and limited resources of the companies.
Without the resources available to large manufacturers, inventors/small companies are often unaware of the long odds they face in developing a viable product. As a result, many promising technologies that could prove beneficial to wheelchair users fail to be commercialized. Those that make it to market rarely have demonstrated usability and effectiveness. As a result, commercial viability may be limited. On the other hand, sometimes inventors/small businesses need to be honestly informed about the limited need or significant limitations of their device. While, this is not the feedback they necessarily want to hear, our experience suggests that they appreciate the unbiased opinion.
Given their lack of resources to support product development, it is not surprising that many small companies and inventors have sought assistance from this and other RERCs over the years. RERCs often have the expertise and capacity that they lack to evaluate technology, construct prototypes, test usability, and develop and transfer products. While this outreach is consistent with the AT Industry report, it also demonstrates that these companies/inventors have a laudable (and well-advised) interest in development and testing of new technologies and products.
This project responds to a need to support AT product development and testing by small companies and individual inventors. It is designed formalize engagement of inventors/small companies and to establish a more formal array of interaction.
Methods
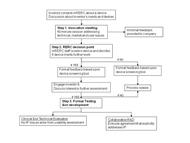
Small companies and inventors who contact the RERC are engaged through a stepwise process. The process is designed to provide a basic level of support and guidance to all inventors/companies with the potential to more fully assist in development and testing of promising devices. The process is illustrated in Figure 1 . Intellectual Property issues are addressed throughout the process to insure that the interests of all parties are judiciously represented.
Step 1: Innovation Meeting.
Inventors present their ideas and obtain feedback from an interdisciplinary group of RERC students, faculty, and clinicians during a 60 minute meeting. The RERC team reviews the product and assesses its potential from three perspectives: technical, market and user. Inventors may attend meeting to present their technologies or a device and/or information may be sent for review. Following the meeting, inventors/companies are provided with a synopsis of the meeting. Typically, Intellectual Property is managed using an Invention Disclosure between the university and the inventor/small company
Step 2: Formal device screening & RERC decision point.
After the Innovation Meeting, the RERC team discusses the new product/technology and performs a more formal analysis of device potential. This device screening assesses the product from both its potential for development and the operational aspects of the RERC to provide assistance. Formal device screening incorporates aspects of the Product Device Manufacturers Association’s (PDMA) Essential Preliminary Analysis that has been modified for AT (as outlined in Table 1).
Product Centric constructs |
Device purpose and function |
Operational constructs |
Current RERC development activity |
Formal screening generates additional written feedback for the inventor/company. This feedback is a more formal follow-up to that following the Innovation Meeting. These reviews addresse product potential and if the RERC has the capacity and expertise to positively impact its development. In other words, the RERC team poses the question: “Does the invention have potential and can we help?” If the answer is NO, device screening information will be compiled and sent to the inventor/company. If the answer is YES, the RERC extends an invitation to continue collaboration. Over the past few years, some inventors/companies have been satisfied with the review provided and chose to continue independently while others have sought additional RERC collaboration. For those inventors/companies that seek additional collaboration, the process moves to Step 3.
Step 3: Formal product testing and/or development.
Two basic options are available:
Clinical and/or technical evaluation.
Most small companies and inventors have little or no capacity to perform user trials or stakeholder assessments. The RERC can organize different levels of user input to better inform the inventor about the design. User trials are appropriate for devices that have been developed to a point of a testable prototype. In cases when only a model or a conceptual design is available, other stakeholder assessment methods can be used, including surveys or focus groups of potential users, caregivers, and/or clinicians.
Relatedly, many companies do not possess the technical capacity needed to assess performance or conduct standardized testing. The RERC can assist in a variety of manners including bench testing of prototypes or assistance in collecting and analyzing data.
Clinical and technical engagement does not impact the Intellectual Property of the device, and the previously executed NDA will protect the inventor.
Collaborative research and development.
In some instances, technical development is necessary to improve or optimize device design. While many inventors and small companies lack R&D capability, the RERC has the engineering capacity to technically develop the products as well as the clinical expertise to perform iterative testing and evaluation. As a result, the collaborative development model enables inventors to engage in joint product development.
The RERC team will work with the inventor/company to define an R&D agreement. These agreements will address both the scope of collaboration and IP issues. This will ensure equitable division of intellectual property between the company and the RERC. The IP of the company will always be the IP of the company. IP developed solely by the university will remain as the university’s IP, and IP jointly developed will be shared.
Outcomes
The following examples are not comprehensive but are presented to provide a synopsis of the different activities and forms of engagement.
Innovation Feedback.
Myriad companies and inventors have presented their designs for quick, multidisciplinary evaluations. The devices reviewed during an Innovation Meeting have included: a scooter modified with an internal combustion engine; an accessible all-terrain vehicle; a patient transfer and transport system; a novel transfer device, a device that configures into a chair and stretcher; a handset that offers hands-free operation, and a ceiling mounted exercise gym.
Technical Assistance:
Criterion Health, a small company in Indiana, received a SBIR grant to develop a novel power mobility base. They sought assistance in defining test methods to illustrate performance for the grant proposal. In addition, the RERC is assisting in the collection and analysis of accelerometer data as a part of their performance evaluation.
Clinical Evaluation:
PostureWorks, a small MA-based company that designs and manufactures low cost seating and positioning products primarily for nursing facilities requested that the RERC perform user testing on its Engage Series Seat Insert. The device is a simple seat insert that is compatible with general use cushions and intended to provide a stable base of support. In response, the RERC conducted testing with residents of a skilled nursing facility to determine the comfort, postural and functional impacts of the design.
Collaborative development.
Cushion by Design is a small, family-owned business in GA that primarily serves the carpet industry. Cushion by Design developed a technology to impregnate silicone into reticulated foam, which they wanted to apply to wheelchair cushions. The impregnated foam is thought to increase the useful life of the foam, permit cleaning or laundering of the cushion, and improve the microclimate of the seating surface (management of temperature and moisture). Cushion by Design collaborated with the RERC to jointly develop wheelchair cushions. The RERC assisted in engineering design (defining the type of reticulated foam to use and the amount and durometer of the silicone that will meet the price points required), and performed bench and human subject testing. Cushion by Design has established relationships with Pride Mobility and PostureWorks to supply this technology for their seating products.
Z Development, a single-person company holding a patent on the use of bladders being deflated by a vacuum to off-load the ischial tuberosities, engaged the RERC to complete technical development and perform clinical testing. This resulted in the design and testing of a dynamic, off-loading wheelchair cushion. License negotiations are currently underway.
References
U.S. Department of Commerce, Technology Assessment of the US Assistive Technology Industry, Office of Strategic Industries and Economic Security Strategic Analysis Division, Editor. 2003
Acknowledgements.
The authors thank the staff of the Rehabilitation Engineering and Applied Research Lab for their assistance in this project and all the inventors who have shared their technologies. This project is supported by the Rehabilitation Engineering Research Center on Wheeled Mobility through the National Institute on Disability and Rehabilitation Research. The comments included in this paper are not necessarily reflective of views held by NIDRR or the Department of Education.